Lead PI:
Center Researchers:
The Challenge
Coronary bifurcation lesions, occurring where a main vessel (MV) divides into a side branch (SB), are particularly difficult to treat due to their complex anatomy and associated hemodynamic disturbances.
These lesions account for approximately 20% of coronary interventions and are prone to complications such as in-stent restenosis, occurring in up to 30% of cases.
While clinicians rely on diagnostic metrics like instantaneous wave-free ratio (iFR) and resting gradient to guide stenting procedures, these measurements are limited in their ability to account for the interplay between lesion curvature, length, and severity.
Existing clinical studies and treatment guidelines are further hindered by a lack of tools that can predict hemodynamic outcomes and inform interventional strategies in real time.
Computational fluid dynamics (CFD) simulations offer detailed hemodynamic insights but remain computationally expensive, requiring significant time and resources incompatible with real-time clinical workflows.
Our Solution
The Randles Lab has developed high-resolution digital twin models to simulate and assess the impact of bifurcation lesion morphology on diagnostic metrics and treatment outcomes.
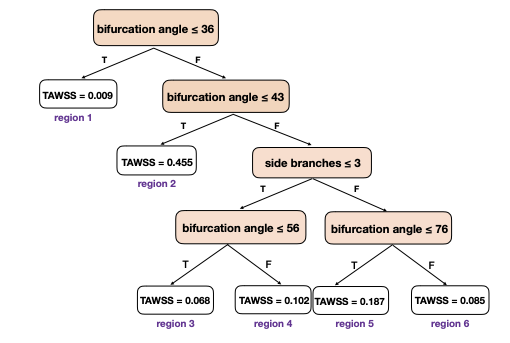 Using patient-derived geometries and massively parallel CFD simulations powered by our HARVEY solver, we model the effects of lesion curvature, length, and severity on clinically relevant metrics such as iFR, resting gradient, and diastolic-systolic velocity ratio (DSVR). These simulations provide unprecedented insights into how specific anatomical features influence blood flow, enabling the identification of high-risk lesions that may lead to interventional complications.
Using patient-derived geometries and massively parallel CFD simulations powered by our HARVEY solver, we model the effects of lesion curvature, length, and severity on clinically relevant metrics such as iFR, resting gradient, and diastolic-systolic velocity ratio (DSVR). These simulations provide unprecedented insights into how specific anatomical features influence blood flow, enabling the identification of high-risk lesions that may lead to interventional complications.
Our approach leverages high performance computing to generate synthetic datasets that can train physics-informed machine learning (ML) models for real-time functional assessment. By analyzing geometric variations of a single patient-specific coronary artery, we have demonstrated that occlusion severity is a key predictor of pressure-based diagnostic changes, while DSVR exhibits greater sensitivity to nuanced morphological changes.
This work bridges the gap between computational accuracy and clinical feasibility by laying the foundation for AI-assisted virtual planning tools that predict hemodynamic outcomes in seconds rather than hours.
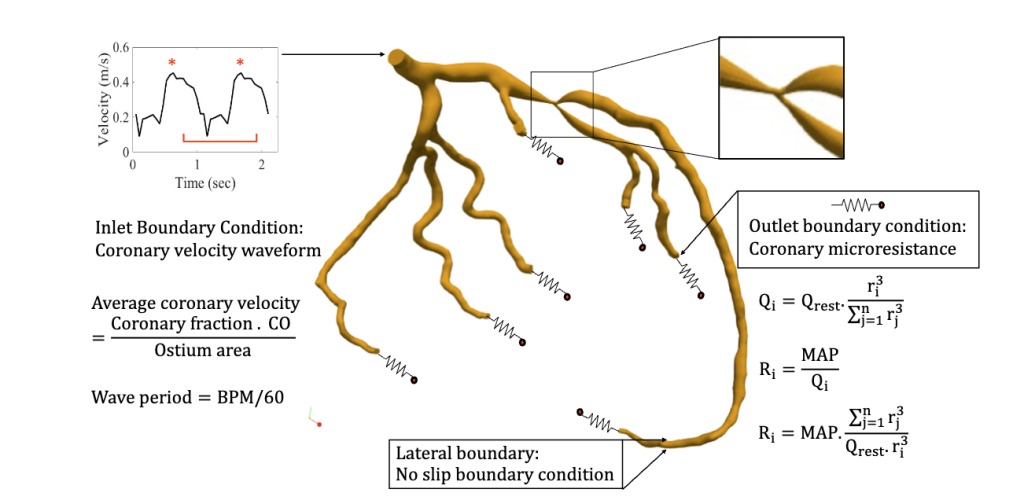
These digital twins can potentially revolutionize bifurcation lesion treatment by providing clinicians with preoperative insights into whether side branch intervention will be necessary following main vessel stenting. Our technology reduces procedural risks and complications and accelerates the adoption of precision medicine approaches in interventional cardiology.
